COVID-19 testing is very much in the news these days. Let’s take a look.
There are two main types of testing: detection of the pathogen (SARS-CoV-2) itself and antibody testing. In detective-speak, the first kind of testing looks for the perpetrator while the second kind of testing looks for the perpetrator’s footprints. The science and machinery are quite different. This article discusses the first kind of test: pathogen detection.
SARS-CoV-2 detection looks for the presence (or absence) of the pathogen’s genetic material. Genetic testing doesn’t try to match a sample against the entire SARS-CoV-2 genome. Instead, genetic tests target specific genes within the genome. It looks for specific sequences within SAR-CoV-2 genetic RNA. Unlike cells which store genetic information in DNA (deoxyribonucleic acid), the SARS-CoV-2 virus stores genetic information in RNA (ribonucleic acid).
The World Health Organization guidance recommends five target sequences which can be used to identify the presence of SARS-CoV-2:
- Nucleocapsid gene (N)
- Envelope gene (E)
- Spike gene (S)
- RNA-dependent RNA polymerase gene (RdRP)
The capsid and envelope genes code for the proteins forming the shell around the virus. The spikes are the proteins protruding from the shell and are the attachment points for cell infection. The RdRP gene codes for the chemical enzyme (polymerase) that assist the replication of virus RNA. The RdRP in this case is specific to SARS-CoV-2. All of these genes are SARS-CoV-2 fingerprints. (See “COVID-19: Know Your Foe.”)
The amount of RNA in a sample is very minute and is not sufficient for immediate chemical analysis. The sample RNA must be amplified, a fancy way of increasing the concentration (amount) of targeted RNA. These kinds of tests are called nucleic acid amplification tests (NAAT).
One form of NAAT is the reverse-transcription polymerase chain reaction (RT-PCR). You may have seen the term “RT-PCR” in the news. RT-PCR is a bread-and-butter technique in genetics research and drug development. Reverse transcrption (RT) turns the RNA into DNA which is amplified using polymerase chain reaction (PCR).
Without deep diving PCR (there are many good on-line tutorials), the term “chain reaction” hints at the technique. We know how an uncontrolled nuclear chain reaction leads to explosive fission. One nucleus splits causing two other nuclei to split and so on. PCR is a chain reaction in which DNA splits into two halves producing two new complete strands of DNA which split into four halves and so on. Each cycle doubles the concentration of the DNA in the sample. Eventually, enough DNA is available in the sample to reliably detect the presence of target genetic sequences.
Real time RT-PCR uses flourescent dyes to reveal the presence of targeted genetic material. Results are produced much faster than older methods employing radioactive isotope markers. Because speed matters, real time RT-PCR is widely used throughout the research and medical communities.
Real time RT-PCR is automated and many manufacturers, like Abbott Molecular, sell machines. Real time RT-PCR machines run a sample through multiple amplification cycles, typically, 30 to 40 cycles. Fluorescence is measured after each cycle. When fluorescence exceeds a threshold, the test is positive, confirming the presence of the targeted genetic sequence (and the pathogen). The machine counts the number of cycles needed to exceed the threshold. Severe infection (i.e., more pathogen RNA in the original sample) requires fewer cycles.
See “How is the COVID-19 Virus Detected using Real Time RT-PCR?”
Abbott M2000 RealTime System
The Abbott M2000 RealTime System is one example of a real time RT-PCR testing machine. The M2000 can test up to 96 samples in one run: 93 patient samples and 3 control samples. Control samples are required to assure valid results. Both positive and negative control samples are required.

The M2000 is a large machine suitable for a laboratory setting.
Like the RT-PCR machines from other vendors, the M2000 requires consumable supplies. Consumable supplies are usually machine-specific. If you own a laser or inkjet printer, you’re already familiar with this concept. 😉 The vendor develops and manufactures pathogen-specific test kits in the form of cartridges, etc. that are physically compatible with their machines.
The Abbott RealTime SARS-CoV-2 EUA test is Abbott’s COVID-19 test for the M2000. “EUA” means “Emergency Use Authorization.” Authorization is issued by the U.S. Food and Drug Administration (FDA). EUA limits the use of the SARS-CoV-2 EUA test and is not full FDA approval. The Abbott RealTime SARS-CoV-2 EUA test is designed specifically for the M2000 real time RT-PCR system for use by authorized laboratories in the U.S.
The M2000 can process up to 470 patient samples in 24 hours, roughly five runs per day, 93 patient samples per run. Abbott planned to ramp up U.S. production to one million tests per week by the end of March. This goal is far short of the millions of tests which experts believe are needed in order to re-open the economy.
Reagents are one of the consumables and you’ve probably heard the term “reagents” in the news as an item which is in short supply. Reagents are one of the main components of the Abbott SARS-CoV-2 test kit. The reagents break open the SAR-CoV-2 virus and release its genetic material (viral RNA). The reagents recognize targeted segments of the SARS-CoV-2 genome, ignoring genetic material from other viruses. The reagents select targeted genes for amplification. The Abbott RealTime SARS-CoV-2 test (assay) targets the RdRP and N genes.
The fine print notes “Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.” Thus, Abbott acknowledges that false negatives are possible.
The M2000 made the news in early April when Dr. Birx (White House coronavirus response coordinator) stated that 80% of the 175 Abbott machines in 120 laboratories across the U.S. were not being fully utilized. The machines are located in academic medical centers and hospital laboratories [Bloomberg News]. I suspect that the bottleneck is collecting samples and getting samples to the central laboratories. New York State plans to have pharmacies collect samples thereby increasing the number of tests per day and improving RT-PCR utilization.
Other big players in the market are Thermo Fisher Scientific (Applied Biosystems TaqPath), Roche )Cobas 6800/8800 System) and BD Molecular (BD MAX System). These tests target other SARS-CoV-2 genes like S and ORF-1AB in addition to N and RdRP.
Abbott ID Now
The Abbott ID NOW™ system is a small machine suitable for a doctor’s office or portable use (8.15″W x 5.7″H x 7.64″D). The Abbott machine was developed originally by Alere. (Abbott acquired Alere in October 2017.) The ID NOW is used to detect the influenza A and B2 viruses as well as COVID-19. ID NOW costs about $12,000USD.
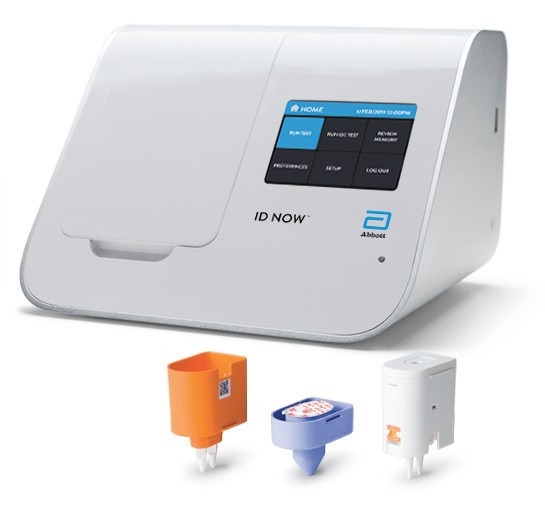
Multi-cycle RT-PCR tests raise and lower the sample temperature during each cycle. ID NOW operates at a single constant temperature, so-called isothermal chemistry. Thus, ID NOW can obtain results faster than conventional real time RT-PCR.
The overall test process is similar. Reagents break open viral RNA in the sample. The reagents recognize the target sequence in the pathogen. The isothermal amplification chemistry amplifies the selected genetic sequence. Fluorescent probes recognize and attach to each replicated sequence. The probe molecules fluoresce. The ID NOW hardware monitors fluorescence. When fluorescence exceeds a trigger threshold, a positive result is displayed.
Like the M2000, it uses consumable supplies like its reagent test cartridge, e.g., the ID NOW COVID-19 assay. A single test costs about $50USD.
The COVID-19 assay targets the SARS-CoV-2 RdRP gene. Results come fast — a positive result in 5 minutes (minimum) or a negative result in 13 minutes. The ID NOW COVID-19 assay is available for use under FDA EUA and is only available in the U.S. The ID NOW COVID-19 test is not yet approved or cleared by the U.S. FDA.
Machines like the Abbott ID NOW™ could greatly speed up COVID-19 testing. Big M2000 machines are centrally located and patient samples must be sent to the lab. The sample may await other samples for a full run. Then results must be reported back to the physician and patient. The round-trip takes too long; a delay of several days is not uncommon. The Abbott ID NOW can be located on site, potentially reducing the round-trip to an hour or two.
The chief disadvantage is cost. The ID NOW single test, cartiridge approach is nore expansive than M2000’s batch testing. ID NOW targets a single SARS-CoV-2 gene, RdRP, making it vulnerable to a mutation in this one gene. The M2000 targets three genes and is less vulnerable to a mutation.
Stay distant and stay healthy — P.J. Drongowski
